 On pense que la cause de la colite ulcéreuse est en quelque sorte liée à des réactions immunologiques anormales du corps aux bactéries normalement présentes dans le côlon.
On pense que la cause de la colite ulcéreuse est en quelque sorte liée à des réactions immunologiques anormales du corps aux bactéries normalement présentes dans le côlon.
Il n'y a aucune preuve clinique ou scientifique qui soutient la théorie selon laquelle un régime alimentaire spécialisé peut causer ou bénéficier aux personnes atteintes de colite ulcéreuse (CU). Cependant, les patients peuvent trouver que certains aliments aggravent les symptômes de la colite ulcéreuse et ils doivent éviter ces aliments. Les symptômes les plus courants de la colite ulcéreuse sont les saignements rectaux, les crampes abdominales et la diarrhée. Certaines personnes recommandent d'éviter un régime riche en fibres (comme les fruits crus, les légumes, les graines, les noix, etc.) en plus d'autres aliments qui aggravent les symptômes. Il peut être raisonnable de tenir un journal alimentaire pour suivre les aliments qui aggravent les symptômes et les aliments qui n'aggravent pas les symptômes (par exemple, les bananes, le riz blanc, le pain blanc, la compote de pommes, les aliments mous fades, etc.) Discutez de vos besoins alimentaires avec votre médecin traitant ou un diététicien spécialisé dans la rectocolite hémorragique et l'alimentation.
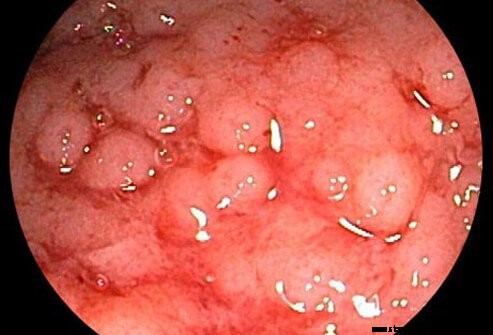 La colite ulcéreuse est considérée comme étant liée à la maladie de Crohn, une autre maladie inflammatoire chronique des intestins (les deux comme maladie intestinale inflammatoire).
La colite ulcéreuse est considérée comme étant liée à la maladie de Crohn, une autre maladie inflammatoire chronique des intestins (les deux comme maladie intestinale inflammatoire).
La colite ulcéreuse est une inflammation chronique du gros intestin (côlon). Le côlon est la partie du système digestif où l'eau est éliminée des matières non digérées et où les déchets restants sont stockés. Le rectum est l'extrémité du côlon adjacente à l'anus. Chez les patients atteints de colite ulcéreuse, les ulcères et l'inflammation de la paroi interne du côlon entraînent des symptômes de douleurs abdominales, de diarrhée et de saignements rectaux.
La colite ulcéreuse est étroitement liée à une autre affection inflammatoire des intestins appelée maladie de Crohn. Ensemble, ils sont souvent appelés maladies inflammatoires de l'intestin (MII). La colite ulcéreuse et les maladies de Crohn sont des maladies chroniques. La maladie de Crohn peut affecter n'importe quelle partie du tractus gastro-intestinal, y compris toutes les couches de la paroi intestinale. Il peut ne pas être limité au tractus gastro-intestinal (affectant le foie, la peau, les yeux et les articulations). La CU n'affecte que la muqueuse du côlon (gros intestin). Les hommes et les femmes sont également touchés. Les premières commencent le plus souvent à l'adolescence et au début de l'âge adulte, mais elles peuvent également commencer pendant l'enfance et plus tard dans la vie.
UC trouvé dans le monde entier, mais est plus fréquent aux États-Unis, en Angleterre et en Europe du Nord. Il est particulièrement fréquent chez les personnes d'origine juive. La colite ulcéreuse est rarement observée en Europe de l'Est, en Asie et en Amérique du Sud, et est rare dans la population noire. Pour des raisons inconnues, une fréquence accrue de cette condition a été observée récemment dans les pays en développement.
Les parents au premier degré de personnes atteintes de colite ulcéreuse ont un risque accru de développer la maladie au cours de leur vie, mais le risque global reste faible.
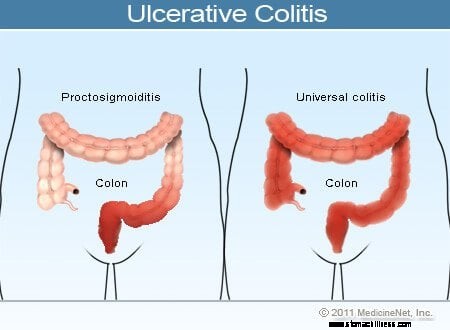 Illustration de la colite ulcéreuse
Illustration de la colite ulcéreuse
Les symptômes courants de la colite ulcéreuse comprennent les saignements rectaux, les douleurs abdominales et la diarrhée, mais il existe un large éventail de symptômes chez les patients atteints de cette maladie. La variabilité des symptômes reflète les différences dans l'étendue de la maladie (la quantité de côlon et de rectum qui sont enflammés) et l'intensité de l'inflammation. Généralement, les patients présentant une inflammation confinée au rectum et un court segment du côlon adjacent au rectum présentent des symptômes plus légers et un meilleur pronostic que les patients présentant une inflammation plus étendue du côlon. Les différents types de rectocolite hémorragique sont classés selon la localisation et l'étendue de l'inflammation :
Alors que l'intensité de l'inflammation du côlon dans la rectocolite hémorragique augmente et diminue avec le temps, la localisation et l'étendue de la maladie chez un patient restent généralement constantes. Par conséquent, lorsqu'un patient atteint de proctite ulcéreuse développe une rechute de sa maladie, l'inflammation est généralement confinée au rectum. Néanmoins, un petit nombre de patients (moins de 10 %) atteints de rectite ulcéreuse ou de proctosigmoïdite peuvent développer ultérieurement une colite plus étendue. Ainsi, les patients qui n'ont initialement qu'une rectite ulcéreuse peuvent développer plus tard une colite du côté gauche ou même une pancolite.


Votre système immunitaire et les
complications de la CU
La cause de la colite ulcéreuse n'est pas connue. À ce jour, il n'y a aucune preuve convaincante qu'elle soit causée par une infection ou qu'elle soit contagieuse.
La colite ulcéreuse implique probablement une activation anormale du système immunitaire dans les intestins. Ce système est censé défendre le corps contre les bactéries nocives, les virus, les champignons et autres envahisseurs étrangers. Normalement, le système immunitaire n'est activé que lorsque le corps est exposé à des envahisseurs nuisibles. Chez les patients atteints de colite ulcéreuse, cependant, le système immunitaire est anormalement et chroniquement activé en l'absence de tout envahisseur connu. L'activation anormale continue du système immunitaire provoque une inflammation chronique et une ulcération des parties du gros intestin. Cette susceptibilité à une activation anormale du système immunitaire est génétiquement héritée. Les parents au premier degré (frères, sœurs, enfants et parents) des patients atteints de MICI sont donc plus susceptibles de développer ces maladies.
Il y a eu plusieurs études utilisant des analyses d'association à l'échelle du génome portant sur la susceptibilité génétique dans la colite ulcéreuse. Ces études ont révélé qu'il y avait environ 30 gènes susceptibles d'augmenter la sensibilité à la colite ulcéreuse, notamment le gène du récepteur d'immunoglobuline FCGR2A, 5p15, 2p16, ORMDL3, ECM1, ainsi que des régions sur les chromosomes 1p36, 12q15, 7q22, 22q13 et IL23R. À ce stade précoce de la recherche, on ne sait toujours pas comment ces associations génétiques seront appliquées au traitement de la maladie, mais elles pourraient avoir des implications futures pour comprendre la pathogenèse et créer de nouveaux traitements.
Le diagnostic de colite ulcéreuse est suggéré par les symptômes de douleur abdominale, de saignement rectal et de diarrhée. Comme il n'y a pas d'étalon-or pour le diagnostic, le diagnostic ultime repose sur une combinaison de symptômes, l'apparence de la muqueuse colique au moment de l'endoscopie, les caractéristiques histologiques des biopsies de la muqueuse colique et des études des selles pour exclure la présence de maladies infectieuses. agents qui peuvent causer l'inflammation.
La connaissance de l'étendue et de la gravité de la colite est importante pour choisir parmi les options de traitement.
Certaines modalités de diagnostic plus récentes comprennent l'endoscopie par capsule vidéo et l'entéroscopie par tomodensitométrie/IRM. L'endoscopie par capsule vidéo (VCE) pourrait être utile pour la détection de la maladie de l'intestin grêle chez les patients ayant reçu un diagnostic de CU avec des caractéristiques atypiques et qui pourraient être suspectés d'avoir effectivement la maladie de Crohn. Avec VCE, les patients avalent une capsule qui contient une caméra qui prend des photos pendant qu'elle se déplace dans les intestins et envoie les images sans fil à un enregistreur. Les photos sont ensuite passées en revue. Dans une étude de 2007, VCE a confirmé la présence d'une maladie de l'intestin grêle chez environ 15 % des patients atteints de rectocolite hémorragique avec des caractéristiques atypiques ou d'une maladie inflammatoire de l'intestin non classée, changeant ainsi le diagnostic en maladie de Crohn (qui ne se limite pas au gros intestin comme dans UC). Cela pourrait être une modalité de diagnostic utile dans cette population de patients spécifique.
La tomodensitométrie et l'IRM entérographie sont des techniques d'imagerie qui utilisent des agents de contraste liquides oraux constitués de solutions de PEG ou de faible concentration de baryum pour fournir une distension plus adéquate du côlon et de l'intestin grêle. Celles-ci se sont avérées supérieures aux techniques d'imagerie standard dans l'évaluation de la pathologie de l'intestin grêle chez les patients atteints de la maladie de Crohn. Il a également été démontré qu'ils fournissent des estimations adéquates de la gravité de la maladie dans la colite ulcéreuse (avec quelques sous-estimations et surestimations).
Les patients atteints de colite ulcéreuse limitée au rectum (proctite) ou de colite limitée à l'extrémité du côlon gauche (proctosigmoïdite) se portent généralement assez bien. De brefs traitements périodiques utilisant des médicaments oraux ou des lavements peuvent être suffisants. Les complications graves sont rares chez ces patients. Chez les personnes atteintes d'une maladie plus étendue, la perte de sang des intestins enflammés peut entraîner une anémie et nécessiter un traitement avec des suppléments de fer ou même des transfusions sanguines. Rarement, le côlon peut se dilater de manière aiguë jusqu'à une grande taille lorsque l'inflammation devient très grave. Cette condition est appelée mégacôlon toxique. Les patients atteints de mégacôlon toxique sont extrêmement malades avec fièvre, douleurs et distension abdominales, déshydratation et malnutrition. À moins que le patient ne s'améliore rapidement avec des médicaments, une intervention chirurgicale est généralement nécessaire pour prévenir la rupture du côlon.
Dans une étude scandinave publiée sur plus de 500 patients atteints de colite ulcéreuse suivis jusqu'à 10 ans après le diagnostic, il a été constaté que leur taux de mortalité ne différait pas de celui de la population générale. En outre, le besoin cumulé de colectomie après 10 ans était de 9,8 %, près de 50 % des patients n'avaient pas rechuté au cours des cinq dernières années de l'étude et seulement 20 % des patients atteints de proctite ou de maladie du côté gauche ont évolué vers une pancolite.
Le cancer du côlon est une complication reconnue de la rectocolite hémorragique chronique. Le risque de cancer commence à augmenter après huit à dix ans de colite. Les patients atteints uniquement de rectite ulcéreuse n'ont probablement pas de risque accru de cancer du côlon par rapport à la population générale. Chez les patients atteints de pancolite active (impliquant tout le côlon) depuis 10 ans ou plus, le risque de cancer du côlon est accru par rapport à la population générale. Chez les patients atteints de colite limitée au côté gauche du côlon, le risque de cancer du côlon est augmenté mais pas aussi élevé que chez les patients atteints de pancolite chronique.
Les patients les plus à risque de cancer sont les patients ayant des antécédents familiaux positifs de cancer du côlon, une longue durée de colite, une atteinte étendue du côlon et une cholangite sclérosante primitive (CSP), une autre complication de la colite ulcéreuse.
Étant donné que ces cancers ont une évolution plus favorable lorsqu'ils sont diagnostiqués et traités à un stade précoce, des examens annuels du côlon peuvent être recommandés après huit ans de maladie étendue connue. Au cours de ces examens, des échantillons de tissus (biopsies) peuvent être prélevés pour rechercher des modifications précancéreuses des cellules qui tapissent le côlon. Lorsque des modifications précancéreuses sont découvertes, l'ablation du côlon peut être nécessaire pour prévenir le cancer du côlon.
Les complications de la colite ulcéreuse peuvent toucher d'autres parties du corps.
Les médicaments et la chirurgie ont été utilisés pour traiter la colite ulcéreuse. Cependant, la chirurgie est réservée aux personnes souffrant d'inflammation grave et de complications potentiellement mortelles. Aucun médicament ne peut guérir la colite ulcéreuse. Les patients atteints de colite ulcéreuse connaîtront généralement des périodes de rechute (aggravation de l'inflammation) suivies de périodes de rémission (résolution de l'inflammation) durant des mois à des années. Pendant les rechutes, les symptômes de douleurs abdominales, de diarrhée et de saignements rectaux s'aggravent. Pendant les rémissions, ces symptômes disparaissent. Les rémissions surviennent généralement en raison d'un traitement médicamenteux ou d'une intervention chirurgicale, mais elles surviennent parfois spontanément, c'est-à-dire sans aucun traitement.
Étant donné que la colite ulcéreuse ne peut être guérie par des médicaments, les objectifs du traitement médicamenteux sont de 1) induire des rémissions, 2) maintenir les rémissions, 3) minimiser les effets secondaires du traitement, 4) améliorer la qualité de vie et 5) minimiser le risque de cancer. . Le traitement de la colite ulcéreuse avec des médicaments est similaire, mais pas toujours identique, au traitement de la maladie de Crohn.
Les médicaments pour le traitement de la colite ulcéreuse comprennent 1) les agents anti-inflammatoires tels que les composés 5-ASA, les corticostéroïdes systémiques, les corticostéroïdes topiques et 2) les immunomodulateurs.
Les médicaments anti-inflammatoires qui diminuent l'inflammation intestinale sont analogues aux médicaments contre l'arthrite qui diminuent l'inflammation des articulations (arthrite). Les médicaments anti-inflammatoires utilisés dans le traitement de la colite ulcéreuse sont :
Les immunomodulateurs sont des médicaments qui suppriment le système immunitaire de l'organisme soit en réduisant les cellules responsables de l'immunité, soit en interférant avec les protéines qui jouent un rôle important dans la promotion de l'inflammation. Les immunomodulateurs deviennent de plus en plus des traitements importants pour les patients atteints de colite ulcéreuse sévère qui ne répondent pas de manière adéquate aux agents anti-inflammatoires. Des exemples d'immunomodulateurs comprennent la 6-mercaptopurine (6-MP), l'azathioprine (Imuran), le méthotrexate (Rheumatrex, Trexall), la cyclosporine (Gengraf, Neoral).
Il a été observé depuis longtemps que le risque de rectocolite hémorragique semble être plus élevé chez les non-fumeurs et chez les ex-fumeurs. Dans certaines circonstances, les patients s'améliorent lorsqu'ils sont traités avec de la nicotine.
Le 5-ASA (acide 5-aminosalicylique), également appelé mésalamine, est chimiquement similaire à l'aspirine. L'aspirine (acide acétylsalicylique) est utilisée depuis de nombreuses années dans le traitement de l'arthrite, de la bursite et de la tendinite (conditions d'inflammation des tissus). L'aspirine, cependant, n'est pas efficace dans le traitement de la colite ulcéreuse. D'autre part, le 5-ASA peut être efficace dans le traitement de la colite ulcéreuse si le médicament peut être administré directement (par voie topique) sur la muqueuse enflammée du côlon. Par exemple, le lavement Rowasa est une solution de 5-ASA qui est efficace dans le traitement de l'inflammation dans et près du rectum (rectite ulcéreuse et rectosigmoïdite ulcéreuse). Cependant, la solution de lavement ne peut pas atteindre une hauteur suffisante pour traiter l'inflammation dans la partie supérieure du côlon. Par conséquent, pour la plupart des patients atteints de colite ulcéreuse, le 5-ASA doit être pris par voie orale. Cependant, lorsque le 5-ASA pur est pris par voie orale, l'estomac et la partie supérieure de l'intestin grêle absorbent la majeure partie du médicament avant qu'il n'atteigne le côlon. Par conséquent, pour être efficace en tant qu'agent oral contre la colite ulcéreuse, le 5-ASA doit être modifié chimiquement pour échapper à l'absorption par l'estomac et les intestins supérieurs. Ces composés 5-ASA modifiés sont la sulfasalazine (Azulfidine), la mésalamine (Pentasa, Rowasa, Asacol, Lialda, Apriso) et l'olsalazine (Dipentum).
La sulfasalazine (azulfidine) est utilisée avec succès depuis de nombreuses années pour induire une rémission chez les patients atteints de colite ulcéreuse légère à modérée. Induire une rémission signifie diminuer l'inflammation intestinale et soulager les symptômes de douleurs abdominales, de diarrhée et de saignements rectaux. La sulfasalazine a également été utilisée pendant de longues périodes pour maintenir les rémissions.
La sulfasalazine est constituée d'une molécule de 5-ASA liée chimiquement à une molécule de sulfapyridine. (La sulfapyridine est un antibiotique sulfamide). La connexion des deux molécules empêche l'absorption par l'estomac et les intestins supérieurs avant d'atteindre le côlon. Lorsque la sulfasalazine atteint le côlon, les bactéries du côlon rompent le lien entre les deux molécules. Après avoir rompu avec le 5-ASA, la sulfapyridine est absorbée dans l'organisme puis excrétée dans l'urine. Cependant, la majeure partie du médicament actif 5-ASA reste dans le côlon pour traiter la colite.
La plupart des effets secondaires de la sulfasalazine sont dus à la molécule de sulfapyridine. Ces effets secondaires comprennent les nausées, les brûlures d'estomac, les maux de tête, l'anémie, les éruptions cutanées et, dans de rares cas, l'hépatite et l'inflammation des reins. Chez les hommes, la sulfasalazine peut réduire le nombre de spermatozoïdes. La réduction du nombre de spermatozoïdes est réversible et le nombre revient généralement à la normale après l'arrêt de la sulfasalazine ou en passant à un autre composé 5-ASA.
Les avantages de la sulfasalazine sont généralement liés à la dose. Par conséquent, des doses élevées de sulfasalazine peuvent être nécessaires pour induire une rémission. Certains patients ne peuvent pas tolérer des doses élevées en raison de nausées et de maux d'estomac. Pour minimiser les maux d'estomac, la sulfasalazine est généralement prise après ou avec les repas. Certains patients trouvent plus facile de prendre de l'azulfidine-EN (forme de sulfasalazine à enrobage entérique). L'enrobage entérique aide à réduire les maux d'estomac. Les nouveaux composés 5-ASA ne contiennent pas de composant sulfapyridine et ont moins d'effets secondaires que la sulfasalazine.
Asacol est un comprimé composé du composé 5-ASA, la mésalamine, entouré d'un revêtement de résine acrylique. (Asacol ne contient pas de sulfamides.) Le revêtement de résine empêche le 5-ASA d'être absorbé lors de son passage dans l'estomac et l'intestin grêle. Lorsque le comprimé atteint l'iléon terminal et le côlon, le revêtement de résine se dissout, libérant ainsi du 5-ASA dans le côlon.
Asacol est efficace pour induire des rémissions chez les patients atteints de rectocolite hémorragique légère à modérée. Il est également efficace lorsqu'il est utilisé pendant des périodes prolongées pour maintenir les rémissions. La dose recommandée d'Asacol pour induire une rémission est de deux comprimés de 400 mg trois fois par jour (total de 2,4 grammes par jour). Deux comprimés d'Asacol deux fois par jour (1,6 grammes par jour) sont recommandés pour maintenir la rémission. Parfois, la dose d'entretien est plus élevée.
Comme pour l'azulfidine, les avantages d'Asacol sont liés à la dose. If patients do not respond to 2.4 grams a day of Asacol, the dose frequently is increased to 3.6 grams a day (and sometimes even higher) to induce remission. If patients fail to respond to the higher doses of Asacol, then alternatives, such as corticosteroids, are considered.
Lialda (mesalamine multi matrix, MMX) is an extended release formulation. It is a 5-ASA medication within an inert matrix that is surrounded by a coating. When the capsule reaches the distal ileum, the outer coating (the capsule) dissolves. The intestinal fluid then is absorbed into the matrix forming a gel-like substance which prolongs the contact of the medication with the colonic wall as the mesalamine slowly separates from the matrix. This extended release formulation allows for higher doses to be taken less frequently throughout the day and might and improve compliance.
The most common side effects experienced while taking Lialda are flatulence, abdominal pain, headache, nausea, and dyspepsia.
Apriso is another formulation of 5-ASA that consists of extended-release mesalamine granules encased in microcrystalline cellulose within a capsule. Dissolution of the capsule occurs in the distal ileum, and, since the granules are encased in the cellulose and only slowly separates from the cellulose, there is prolonged delivery of medication as the cellulose and mesalamine travel through the colon.
The most common side effects of this medication are headache, diarrhea, abdominal pain, nausea, nasopharyngitis, influenza-like illness, and sinusitis.
Pentasa is a capsule consisting of the 5-ASA compound mesalamine inside controlled-release spheres. Like Asacol, it is sulfa free. As the capsule travels down the intestines, the 5-ASA inside the spheres is slowly released into the intestines. Unlike Asacol, the mesalamine in Pentasa is released into the small intestine as well as the colon. Therefore, Pentasa can be effective in treating inflammation in the small intestine and the colon. Pentasa is currently the most logical 5-ASA compound for treating mild to moderate Crohn's disease involving the small intestine. Pentasa also is used to induce remission and maintain remission among patients with mild to moderate ulcerative colitis.
Olsalazine (Dipentum) consists of two 5-ASA molecules linked together. It is sulfa free. The linked 5-ASA molecules travel through the stomach and the small intestine unabsorbed. When the drug reaches the terminal ileum and the colon, the normal bacteria in the intestine break the linkage and release the active drug into the colon and the terminal ileum. Olsalazine has been used in treating ulcerative colitis and in maintaining remissions. A side effect unique to olsalazine is secretory diarrhea (diarrhea resulting from excessive production of fluid in the intestines). This condition occurs in some patients, and the diarrhea sometimes can be severe.
Balsalazide (Colazal) is a capsule in which the 5-ASA is linked by a chemical bond to another molecule that is inert (without effect on the intestine) and prevents the 5-ASA from being absorbed. This drug is able to travel through the intestine unchanged until it reaches the end of the small bowel (terminal ileum) and colon. There, intestinal bacteria break apart the 5-ASA and the inert molecule, releasing the 5-ASA. Because intestinal bacteria are most abundant in the terminal ileum and colon, Colazal is used to treat inflammation predominantly localized to the colon.
More clinical trials are needed to compare the effectiveness of Colazal to the other mesalamine compounds such as Asacol in treating ulcerative colitis. Therefore in the United States, choosing which 5-ASA compound has to be individualized. Some doctors prescribe Colazal for patients who cannot tolerate or fail to respond to Asacol. Others prescribe Colazal for patients with predominantly left sided colitis, since some studies seem to indicate that Colazal is effective in treating left sided colitis.
The sulfa-free 5-ASA compounds have fewer side effects than sulfasalazine and also do not impair male fertility. In general, they are safe medications for long-term use and are well-tolerated.
Patients allergic to aspirin should avoid 5-ASA compounds because they are chemically similar to aspirin.
Rare kidney inflammation has been reported with the use of 5-ASA compounds. These compounds should be used with caution in patients with known kidney disease. It also is recommended that blood tests of kidney function be obtained before starting and periodically during treatment.
Rare instances of acute worsening of diarrhea, cramps, and abdominal pain may occur which is at times may be accompanied by fever, rash, and malaise. This reaction is believed to represent an allergy to the 5-ASA compound.
Rowasa is the 5-ASA compound mesalamine in enema form and is effective in ulcerative proctitis and ulcerative proctosigmoiditis (two conditions where active 5-ASA drugs taken as enemas can easily reach the inflamed tissues directly). Each Rowasa enema contains 4 grams of mesalamine in 60 cc of fluid. The enema usually is administered at bedtime, and patients are encouraged to retain the enema through the night.
The enema contains sulfite and should not be used by patients with sulfite allergy. Otherwise, Rowasa enemas are safe and well-tolerated.
Rowasa also comes in suppository form for treating limited proctitis. Each suppository contains 500 mg of mesalamine and usually is administered twice daily.
While some patients improve within several days of starting Rowasa, the usual course of treatment is three to six weeks. Some patients may need even longer courses of treatment for optimal benefit. In patients who do not respond to Rowasa, oral 5-ASA compounds (such as Asacol) can be added. Some studies have reported increased effectiveness in treating ulcerative proctitis and proctosigmoiditis by combining oral 5-ASA compounds with Rowasa enemas. Oral 5-ASA compounds also are used to maintain remission in ulcerative proctitis and proctosigmoiditis.
Another alternative for patients who fail to respond to Rowasa or who cannot use Rowasa is cortisone enemas (Cortenema). Cortisone is a potent anti-inflammatory agent. Oral corticosteroids are systemic drugs with serious and predictable long-term side effects. Cortenema is a topical corticosteroid that has less absorption into the body than oral corticosteroids, and, therefore, it has fewer and less severe side effects.
Corticosteroids (Prednisone, prednisolone, hydrocortisone, etc.) have been used for many years in the treatment of patients with moderate to severe Crohn's disease and ulcerative colitis or who fail to respond to optimal doses of 5-ASA compounds. Unlike the 5-ASA compounds, corticosteroids do not require direct contact with the inflamed intestinal tissues to be effective. Oral corticosteroids are potent anti-inflammatory agents. After absorption, corticosteroids exert prompt anti-inflammatory action throughout the body. Consequently, they are used in treating Crohn's enteritis, ileitis, and ileocolitis, as well as ulcerative and Crohn's colitis. In critically ill patients, intravenous corticosteroids (such as hydrocortisone) can be given in the hospital.
Corticosteroids are faster acting than the 5-ASA compounds. Patients frequently experience improvement in their symptoms within days of starting corticosteroids. Corticosteroids, however, do not appear to be useful in maintaining remissions in ulcerative colitis.
Side effects of corticosteroids depend on the dose and duration of use. Short courses of prednisone, for example, usually are well tolerated with few and mild side effects. Long term, high doses of corticosteroids usually produce predictable and potentially serious side effects. Common side effects include rounding of the face (moon face), acne, increased body hair, diabetes, weight gain, high blood pressure, cataracts, glaucoma, increased susceptibility to infections, muscle weakness, depression, insomnia, mood swings, personality changes, irritability, and thinning of the bones (osteoporosis) with an accompanying increased risk of compression fractures of the spine. Children on corticosteroids can experience stunted growth.
The most serious complication from long term corticosteroid use is aseptic necrosis of the hip joints. Aseptic necrosis means death of bone tissue. It is a painful condition that can ultimately lead to the need for surgical replacement of the hips. Aseptic necrosis also has been reported in knee joints. It is unknown how corticosteroids cause aseptic necrosis. Patients on corticosteroids who develop pain in the hips or knees should report the pain to their doctors promptly. Early diagnosis of aseptic necrosis with cessation of corticosteroids has been reported in some patients to decrease the severity of the condition and possibly help avoid hip replacement.
Prolonged use of corticosteroids can depress the ability of the body's adrenal glands to produce cortisol (a natural corticosteroid necessary for proper functioning of the body). Abruptly discontinuing corticosteroids can cause symptoms due to a lack of natural cortisol (a condition called adrenal insufficiency). Symptoms of adrenal insufficiency include nausea, vomiting, and even shock. Withdrawing corticosteroids too quickly also can produce symptoms of joint aches, fever, and malaise. Therefore, corticosteroids need to be gradually reduced rather than abruptly stopped.
Even after the corticosteroids are discontinued, the adrenal glands' ability to produce cortisol can remain depressed for months to two years. The depressed adrenal glands may not be able to produce enough cortisol to help the body handle stress such as accidents, surgery, and infections. These patients will need treatment with corticosteroids (prednisone, hydrocortisone, etc.) during stressful situations to avoid developing adrenal insufficiency.
Because corticosteroids are not useful in maintaining remission in ulcerative colitis and Crohn's disease and because they have predictable and potentially serious side effects, these drugs should be used for the shortest possible length of time.
Once the decision is made to use oral corticosteroids, treatment usually is initiated with prednisone, 40-60 mg daily. The majority of patients with ulcerative colitis respond with an improvement in symptoms. Once symptoms improve, prednisone is reduced by 5-10 mg per week until the dose of 20 mg per day is reached. The dose then is tapered at a slower rate until the prednisone ultimately is discontinued. Gradually reducing corticosteroids not only minimizes the symptoms of adrenal insufficiency, it also reduces the chances of abrupt relapse of the colitis.
Many doctors use 5-ASA compounds at the same time as corticosteroids. In patients who achieve remission with systemic corticosteroids, 5-ASA compounds such as Asacol are often continued to maintain remissions.
In patients whose symptoms return during reduction of the dose of corticosteroid, the dose of corticosteroids is increased slightly to control the symptoms. Once the symptoms are under control, the reduction can resume at a slower pace. Some patients become corticosteroid dependent. These patients consistently develop symptoms of colitis whenever the corticosteroid dose reaches below a certain level. In patients who are corticosteroid dependent or who are unresponsive to corticosteroids, other anti-inflammatory medications, immunomodulator medications or surgery are considered.
The management of patients who are corticosteroid dependent or patients with severe disease which responds poorly to medications is complex. Doctors who are experienced in treating inflammatory bowel disease and in using the immunomodulators should evaluate these patients.
Long-term use of corticosteroids such as prednisolone or prednisone can cause osteoporosis . Corticosteroids cause decreased calcium absorption from the intestines and increased loss of calcium from the kidneys and bones. Increasing dietary calcium intake is important but alone cannot halt corticosteroid-induced bone loss. Management of patients on long term corticosteroids should include:
Oral budesonide (Entocort EC) is a topically acting corticosteroid which was been shown to be effective in Crohn's disease, and in enema formulation for left-sided ulcerative colitis with fewer side effects than oral steroids. In a recent meta-analysis, however, it was found to be significantly less likely to induce clinical remission in patients with ulcerative colitis than oral mesalamine after 8 weeks of therapy. Therefore, use of this medication is not recommended at this time to treat flares of ulcerative colitis.
Golimumab is an injectable man-made protein that binds to tumor necrosis factor alpha in the body, and blocks the effects of tumor necrosis factor alfa in patients with ulcerative colitis. Golimumab is injected under the skin, and injection sites should be rotated. Golimumab interacts with several drugs and has several side effects. Consult with your prescribing physician or pharmacist to discuss these potential drug interactions and side effects.
Immunomodulators are medications that weaken the body's immune system. The immune system is composed of immune cells and the proteins that these cells produce. These cells and proteins serve to defend the body against harmful bacteria, viruses, fungi, and other foreign invaders. Activation of the immune system causes inflammation within the tissues where the activation occurs. (Inflammation is, in fact, an important mechanism to defend the body used by the immune system.) Normally, the immune system is activated only when the body is exposed to harmful invaders. In patients with Crohn's disease and ulcerative colitis, however, the immune system is abnormally and chronically activated in the absence of any known invader. Immunomodulators decrease tissue inflammation by reducing the population of immune cells and/or by interfering with their production of proteins that promote immune activation and inflammation. Generally, the benefits of controlling moderate to severe ulcerative colitis outweigh the risks of infection due to weakened immunity. Examples of immunomodulators include azathioprine (Imuran), 6-mercaptopurine (6-MP, Purinethol), cyclosporine (Sandimmune), and methotrexate (Rheumatrex, Trexall).
Azathioprine and 6-mercaptopurine (6-MP) are medications that weaken the body's immunity by reducing the population of a class of immune cells called lymphocytes. Azathioprine and 6-MP are related chemically. Specifically, azathioprine is converted into 6-MP inside the body. In high doses, these two drugs have been useful in preventing rejection of transplanted organs and in treating leukemia. In low doses, they have been used for many years to treat patients with moderate to severe Crohn's disease and ulcerative colitis.
Azathioprine and 6-MP are increasingly recognized by doctors as valuable drugs in treating Crohn's disease and ulcerative colitis. Some 70% of patients with moderate to severe disease will benefit from these drugs. Because of the slow onset of action and the potential for side effects, however, 6-MP and azathioprine are used mainly in the following situations:
When azathioprine and 6-MP are added to corticosteroids in the treatment of ulcerative colitis patients who do not respond to corticosteroids alone, there may be an improved response or smaller doses and shorter courses of corticosteroids may be effective. Some patients can discontinue corticosteroids altogether without experiencing relapses. The ability to reduce corticosteroid requirements has earned 6-MP and azathioprine their reputation as "steroid-sparing" medications.
In patients with severe ulcerative colitis who suffer frequent relapses, 5-ASA may not be sufficient, and more potent azathioprine and 6-MP will be necessary to maintain remissions. In the doses used for treating ulcerative colitis and Crohn's disease, the long-term side effects of azathioprine and 6-MP are less serious than long-term oral corticosteroids or repeated courses of oral corticosteroids.
Side effects of 6-MP and azathioprine include increased vulnerability to infections, inflammation of the liver (hepatitis) and pancreas, (pancreatitis), and bone marrow toxicity (interfering with the formation of cells that circulate in the blood).
The goal of treatment with 6-MP and azathioprine is to weaken the body's immune system in order to decrease the intensity of inflammation in the intestines; however, weakening the immune system increases the patient's vulnerability to infections. For example, in a group of patients with severe Crohn's disease unresponsive to standard doses of azathioprine, raising the dose of azathioprine helped to control the disease, but two patients developed cytomegalovirus (CMV) infection. CMV usually infects individuals with weakened immune systems such as patients with AIDS or cancer, especially if they are receiving chemotherapy, which further weakens the immune system.
Azathioprine and 6-MP-induced inflammation of the liver (hepatitis) and pancreas (pancreatitis) are rare. Pancreatitis typically causes severe abdominal pain and sometimes vomiting. Pancreatitis due to 6-MP or azathioprine occurs in a small percentage of patients, usually during the first several weeks of treatment. Patients who develop pancreatitis should not receive either of these two medications again.
Azathioprine and 6-MP also suppress the bone marrow. The bone marrow is where red blood cells, white blood cells, and platelets are made. Actually, a slight reduction in the white blood cell count during treatment is desirable since it indicates that the dose of 6-MP or azathioprine is high enough to have an effect; however, excessively low red or white blood cell counts indicates bone marrow toxicity. Therefore, patients on 6-MP and azathioprine should have periodic blood counts (usually every two weeks initially and then every 3 months during maintenance) to monitor the effect of the drugs on their bone marrow.
6-MP can reduce the sperm count in men. When the partners of male patients on 6-MP conceive, there is a higher incidence of miscarriages and vaginal bleeding. There also are respiratory difficulties in the newborn. Therefore, it is recommended that whenever feasible, male patients should stop 6-MP and azathioprine for three months before attempting to conceive.
Patients on long-term, high dose azathioprine to prevent rejection of the kidney after kidney transplantation have an increased risk of developing lymphoma, a malignant disease of lymphatic cells. There is no evidence at present that long term use of azathioprine and 6-MP in the low doses used in IBD increases the risk for lymphoma, leukemia or other malignancies.
One problem with 6-MP and azathioprine is their slow onset of action. Typically, full benefit of these drugs is not realized for three months or longer. During this time, corticosteroids frequently have to be maintained at high levels to control inflammation.
The reason for this slow onset of action is partly due to the way doctors prescribe 6-MP. Typically, 6-MP is started at a dose of 50 mg daily. The blood count is then checked two weeks later. If the white blood cell count (specifically the lymphocyte count) is not reduced, the dose is increased. This cautious, stepwise approach helps prevent severe bone marrow and liver toxicity, but also delays benefit from the drug.
Studies have shown that giving higher doses of 6-MP early can speed up the benefit of 6-MP without increased toxicity in most patients, but some patients do develop severe bone marrow toxicity. Therefore, the dose of 6-MP has to be individualized. Scientists now believe that an individual's vulnerability to 6-MP toxicity is genetically inherited. Blood tests can be performed to identify those individuals with increased vulnerability to 6-MP toxicity. In these individuals, lower initial doses can be used. Blood tests can also be performed to measure the levels of certain by-products of 6-MP. The levels of these by-products in the blood help doctors more quickly determine whether the dose of 6-MP is right for the patient.
Azathioprine is converted into 6-MP in the body, and 6-MP then is partially converted into other chemicals by an enzyme called thiopurine methyltransferase (TPMT). These chemicals then are eliminated from the body. The activity of TPMT that determines the ability to convert 6-MP into other chemicals is genetically determined, and approximately 10% of the population in the United States has reduced or absent TPMT activity. In this 10% of patients, 6-MP and another related chemical (6-thioguanine or 6-TG) accumulate and are toxic to the bone marrow where blood cells are produced. Thus, when given normal doses of azathioprine or 6-MP, these patients with reduced or absent TPMT activity can develop seriously low white blood cell counts for prolonged periods of time, exposing them to serious life-threatening infections.
The Food and Drug Administration now recommends that doctors check TPMT levels prior to starting treatment with azathioprine or 6-MP. Patients found to have genes associated with reduced or absent TPMT activity are treated with alternative medications or are prescribed substantially lower than normal doses of 6-MP or azathioprine.
A word of caution is in order, however. Having normal TPMT genes is no guarantee against azathioprine or 6-MP toxicity. Rarely, a patient with normal TPMT genes can develop severe toxicity in the bone marrow and a low white blood cell count even with normal doses of 6-MP or azathioprine. In addition, with normal levels of TPMT activity, liver toxicity, another toxic effect of 6-MP, can still occur. Therefore, all patients taking 6-MP or azathioprine (regardless of TPMT genetics) have to be closely monitored by a doctor who will order periodic blood counts, and liver enzyme tests for as long as the medication is taken.
Another cautionary note:allopurinol (Zyloprim), used in treating high blood uric acids levels and gout, can induce bone marrow toxicity when used together with azathioprine or 6-MP. This occurs because allopurinol reduces TMPT activity. The combination of genetically-reduced TMPT activity and further reduction of TMPT activity by the allopurinol increases greatly the risk of bone marrow toxicity.
In addition to monitoring blood cell counts and liver tests, doctors also may measure blood levels of the chemicals that are formed from 6-MP (6-MP metabolites), which can be helpful in several situations such as:
Patients have been maintained on 6-MP or azathioprine for years without any important long-term side effects. Their doctors, however, should closely monitor their patients on long-term 6-MP. There is data suggesting that patients on long-term maintenance with 6-MP or azathioprine fare better than those who stop these medications. Those who stop 6-MP or azathioprine are more likely to experience relapses, more likely to need corticosteroids or undergo surgery.
Methotrexate (Rheumatrex, Trexall) is an immunomodulator and anti-inflammatory medication. Methotrexate has been used for many years in the treatment of severe rheumatoid arthritis and psoriasis. It has been helpful in treating patients with moderate to severe Crohn's disease who are either not responding to 6-MP and azathioprine or are intolerant of these two medications. Methotrexate also may be effective in patients with moderate to severe ulcerative colitis who are not responding to corticosteroids or 6-MP and azathioprine. It can be given orally or by weekly injections under the skin or into the muscles. It is more reliably absorbed with the injections.
One major complication of methotrexate is the development of liver cirrhosis when the medication is given over a prolonged period of time (years). The risk of liver damage is higher in patients who also abuse alcohol or have morbid (severe) obesity. Generally, periodic liver biopsies are recommended for a patient who has received a cumulative (total) methotrexate dose of 1.5 grams and higher.
Other side effects of methotrexate include low white blood cell counts and inflammation of the lungs.
Methotrexate should not be used in pregnancy.
Cyclosporine (Sandimmune) is a potent immunosuppressant used in preventing organ rejection after transplantation, for example, of the liver. It also has been used to treat patients with severe ulcerative colitis and Crohn's disease. Because of the approval of infliximab (Remicade) for treating severe Crohn's disease, cyclosporine probably will be used primarily in severe ulcerative colitis. Cyclosporine is useful in fulminant ulcerative colitis and in severely ill patients who are not responding to systemic corticosteroids. Administered intravenously, cyclosporine can be very effective in rapidly controlling severe colitis and avoiding or delaying surgery.
Cyclosporine also is available as an oral medication, but the relapse rate with oral cyclosporine is high. Therefore, cyclosporine seems most useful when administered intravenously in acute situations.
Side effects of cyclosporine include high blood pressure, impairment of kidney function, and tingling sensations in the extremities. More serious side effects include anaphylactic shock and seizures.
Infliximab (Remicade) is an antibody that attaches to a protein called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha is one of the proteins produced by immune cells during activation of the immune system. TNF-alpha, in turn, stimulates other cells of the immune system to produce and release other proteins that promote inflammation. In Crohn's disease and in ulcerative colitis, there is continued production of TNF-alpha as part of the immune activation. Infliximab, by attaching to TNF-alpha, blocks its activity and in so doing decreases the inflammation.
Infliximab, an antibody to TNF-alpha, is produced by the immune system of mice after the mice are injected with human TNF-alpha. The mouse antibody then is modified to make it look more like a human antibody, and this modified antibody is infliximab. Such modifications are necessary to decrease the likelihood of allergic reactions when the antibody is administered to humans. Infliximab is given by intravenous infusion over two hours. Patients are monitored throughout the infusion for side effects.
Infliximab has been used effectively for many years for the treatment of moderate to severe Crohn's disease that was not responding to corticosteroids or immuno-modulators. In Crohn's disease patients, a majority experienced improvement in their disease after one infusion of infliximab. Some patients noticed improvement in symptoms within days of the infusion. Most patients experienced improvement within two weeks. In patients who respond to infliximab, the improvements in symptoms can be dramatic. Moreover, there can be impressively rapid healing of the ulcers and the inflammation in the intestines after just one infusion.
Only over the last few years infliximab also has been used to treat severe UCs. In a study of over 700 patients with moderate to severe UC, for example, infliximab was found to be more effective than placebo in inducing and maintaining remission.
Infliximab is typically given to induce remission in three doses - at time zero and then two weeks and four weeks later. After remission is attained, maintenance doses can be given every other month.
Infliximab, generally, is well tolerated. There have been rare reports of side effects during infusions, including chest pain, shortness of breath, and nausea. These effects usually resolve spontaneously within minutes if the infusion is stopped. Other commonly reported side effects include headache and upper respiratory tract infection.
Infliximab, like immuno-modulators, increases the risk for infection. One case of salmonella colitis and several cases of pneumonia have been reported with the use of infliximab. There also have been cases of tuberculosis (TB) reported after the use of infliximab.
Because infliximab is partly a mouse protein, it may induce an immune reaction when given to humans, especially with repeated infusions. In addition to the side effects that occur while the infusion is being given, patients also may develop a "delayed allergic reaction" that occurs 7-10 days after receiving the infliximab. This type of reaction may cause flu-like symptoms with fever, joint pain and swelling, and a worsening of Crohn's disease symptoms. It can be serious, and if it occurs, a physician should be contacted. Paradoxically, those patients who have more frequent infusions of Remicade are less likely to develop this type of delayed reaction compared to those patients who receive infusions separated by long intervals (6-12 months).
There are some reports of worsening heart disease in patients who have received Remicade. The precise mechanism and role of infliximab in the development of this side effect is unclear. As a precaution, individuals with heart disease should inform their physician of this condition before receiving infliximab.
There have been rare reports of nerve damage such as optic neuritis (inflammation of the nerve of the eye) and motor neuropathy (damage to the nerves controlling muscles).
There have also been rare reports of patients developing viral colitis (cytomegalovirus and herpes simplex virus) while on immunosuppressive medications. These viral infections can mimic a flare of ulcerative colitis and mistakenly suggest resistance to therapy. Before increasing the dose or changing the medication being used to treat the ulcerative colitis, patients should have a thorough evaluation including flexible sigmoidoscopy or limited colonoscopy with biopsies to help make the diagnosis of viral colitis.
Infliximab can aggravate and cause the spread of an existing infection. Therefore, it should not be given to patients with pneumonia, urinary tract infection or abscess (localized collection of pus).
It now is recommended that patients be tested for TB prior to receiving infliximab. Patients who previously had TB should inform their physician of this before they receive infliximab.
Infliximab can cause the spread of cancer cells; therefore, it should not be given to patients with cancer.
The effects of infliximab on the fetus are not known although the literature suggests that this medication is safe for women to continue until week 32 of pregnancy. At that time, the risk of exposure of the fetus to this medication by placental transfer is increased. Infliximab is listed as a pregnancy category B drug by the FDA (meaning there has been no documented human toxicity).
Because infliximab is partly a mouse protein, some patients can develop antibodies against infliximab with repeated infusions. The development of these antibodies can decrease the effectiveness of the drug. The chances of developing these antibodies can be decreased by concomitant use of 6-MP and corticosteroids. There are ongoing studies in patients who have lost their initial response to infliximab to determine whether measurement of antibody titers will be helpful in guiding further treatment. Results of these studies are not yet available.
While infliximab represents an exciting new class of medications in the fight against Crohn's disease and ulcerative colitis, caution is warranted because of potentially serious side effects. Doctors are using infliximab in moderate to severe ulcerative colitis not responding to other medications.
Adalimumab is an anti-TNF drug similar to infliximab. It decreases inflammation by blocking tumor necrosis factor (TNF-alpha). In contrast to infliximab, adalimumab is a fully humanized anti-TNF antibody containing no mouse protein and, therefore, might cause less of an immune reaction. Adalimumab is administered subcutaneously (under the skin) instead of intravenously as in the case of infliximab.
Rheumatologists have been using adalimumab for treating inflammation of the joints in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. It was also approved by the FDA in 2007 for the treatment of moderately to severely active Crohn's disease. Though not approved formally by the FDA for treatment of ulcerative colitis, a few studies have shown it to have some efficacy in treating patients with ulcerative colitis who are refractory to or have lost their response to infliximab. More information will be required before recommending this as a standard therapy.
Visilizumab is a humanized antibody that specifically binds to human CD3 expressing T cells, that inhibits the activity of the cells. (CD3 expressing T cells are part of the immune system and seem to play an important role in promoting the inflammation of ulcerative colitis.). In a phase 1 open-label study evaluating the safety and tolerability of this medication, 32 subjects received visilizumab. Results showed that 84% of these patients achieved a clinical response by day 30, 41% achieved clinical remission, and 44% achieved endoscopic remission. Main side effects were decreased CD4 counts and cytokine release syndromes (flu-like symptoms, etc), though there were no serious infections. Initial data seems promising though more must be learned about this medication before it can be used routinely. This medication is not yet approved by the FDA for treatment of ulcerative colitis.
Alpha-4 integrins on the surface of cells of the immune system help the cells to leave the blood and travel into the tissues where they promote inflammation. Antibodies against these integrins have been developed, to dampen the inflammatory response. Natalizumab is one such agent, and in small studies in patients with ulcerative colitis has been shown to have some efficacy in leading to clinical remission. Another more gut-selective humanized antibody (MLN02) has been evaluated in multi-center trials and has also been found to lead to clinical and endoscopic remission in more patients than placebo. More studies must be conducted to evaluate long term effectiveness and side effects of these medications. This medication is not yet approved by the FDA for treatment of ulcerative colitis.
Surgery for ulcerative colitis usually involves removing the entire colon and the rectum. Removal of the colon and rectum is the only permanent cure for ulcerative colitis. This procedure also eliminates the risk of developing colon cancer. Surgery in ulcerative colitis is reserved for the following patients:
Standard surgery involves the removal of the entire colon, including the rectum. A small opening is made in the abdominal wall and the end of the small intestine is attached to the skin of the abdomen to form an ileostomy. Stool collects in a bag that is attached over the ileostomy. Recent improvements in the construction of ileostomies have allowed for continent ileostomies. A continent ileostomy is a pouch created from the intestine. The pouch serves as a reservoir similar to a rectum, and is emptied on a regular basis with a small tube. Patients with continent ileostomies do not need to wear collecting bags.
More recently, a surgery has been developed which allows stool to be passed normally through the anus. In an ileo-anal anastomosis, the large intestine is removed and the small intestine is attached just above the anus. Only the diseased lining of the anus is removed and the muscles of the anus remain intact. In this "pull-through" procedure, the normal route of stool elimination is maintained. This procedure has a relatively good success rate, although pouchitis (inflammation of the distal ileum now acting as the rectum) is a well known complication (that should be confirmed by endoscopy) that is manifested by increased diarrhea, urgency, bleeding, and pain.
For refractory cases, oral steroids or IV infliximab can be used (though this is less well studied in distal colitis)
Also, surgery is recommended for severe colitis refractory to medical therapy
Although it seems plausible that a specialized diet might benefit patients with ulcerative colitis, there is actually no evidence to support treatment with dietary modification. Despite extensive research, no diet has been found to slow progression, treat, or cure the disease. It is recommended that patients stay on a balanced, healthy diet rich in fruits, vegetables, grains, lean meats, beans, fish, eggs, nuts. Patients should also try to limit foods with saturated fats high cholesterol. During flare-ups, patients should continue to eat as tolerated. The Crohn's and Colitis Foundation of America recommends a bland diet with soft food during a flare including hot cereals, boiled eggs, mashed potatoes, steamed vegetables, canned or cooked vegetables to minimize discomfort.
Active research is also ongoing to find other biological agents that are potentially more effective with fewer side effects in treating ulcerative colitis including adalimumab, visilizumab, and alpha-4 integrin blockers.
Research in ulcerative colitis is very active, and many questions remain to be answered. The cause, mechanism of inflammation, and optimal treatments have yet to be defined. Researchers have recently identified genetic differences among patients which may allow them to select certain subgroups of patients with ulcerative colitis who may respond differently to medications. Newer and safer medications are being developed. Improvements in surgical procedures to make them safer and more effective continue to emerge.
It is recommended that adults with inflammatory bowel disease generally follow the same vaccination schedules as the general population.
Osteoporosis has also increasingly been recognized as a significant health problem in patients with IBD. IBD patients tend to have markedly reduced bone mineral densities. Screening with a bone density study is recommended in:
For this reason, most patients with IBD should be on calcium and vitamin D supplementation.
 Les coronavirus humains ont besoin de matériaux organiques pour se transférer efficacement entre les surfaces
Les coronavirus humains ont besoin de matériaux organiques pour se transférer efficacement entre les surfaces
 Œsophage de Barrett
Œsophage de Barrett
 Une variante génétique commune explique pourquoi l'immunothérapie échoue souvent dans la maladie de Crohn
Une variante génétique commune explique pourquoi l'immunothérapie échoue souvent dans la maladie de Crohn
 Les 6 meilleurs livres sur l'intestin et les livres sur la santé que vous devez lire en 2020
Les 6 meilleurs livres sur l'intestin et les livres sur la santé que vous devez lire en 2020
 La soupe au poulet SCD Intro Diet :comment bien la préparer et pourquoi la manger !
La soupe au poulet SCD Intro Diet :comment bien la préparer et pourquoi la manger !
 Le vinaigre de cidre fonctionne-t-il pour le SII ?
Le vinaigre de cidre fonctionne-t-il pour le SII ?
 Le lien entre le dysfonctionnement du plancher pelvien et les symptômes du SII
Existe-t-il un lien entre le dysfonctionnement du plancher pelvien (PFD) et le syndrome du côlon irritable (IBS) ? Le PFD est une condition dans laquelle les muscles du plancher pelvien saffaiblissent
Le lien entre le dysfonctionnement du plancher pelvien et les symptômes du SII
Existe-t-il un lien entre le dysfonctionnement du plancher pelvien (PFD) et le syndrome du côlon irritable (IBS) ? Le PFD est une condition dans laquelle les muscles du plancher pelvien saffaiblissent
 Comment durcir les piles ?
Que sont les piles ? Dans la plupart des cas, vous pouvez gérer les symptômes des piles à la maison. Parfois, vous avez besoin dune intervention chirurgicale pour réparer les pieux. Les piles s
Comment durcir les piles ?
Que sont les piles ? Dans la plupart des cas, vous pouvez gérer les symptômes des piles à la maison. Parfois, vous avez besoin dune intervention chirurgicale pour réparer les pieux. Les piles s
 Le triste état de la médecine américaine :pourquoi les patients et les praticiens souffrent (et comment y remédier)
Jai écrit des articles de blog désagréables, affreux et carrément méchants… Et les a supprimés le lendemain. Vous ne les lirez jamais. Ils venaient dun lieu de souffrance et de douleur. Ils nallaie
Le triste état de la médecine américaine :pourquoi les patients et les praticiens souffrent (et comment y remédier)
Jai écrit des articles de blog désagréables, affreux et carrément méchants… Et les a supprimés le lendemain. Vous ne les lirez jamais. Ils venaient dun lieu de souffrance et de douleur. Ils nallaie